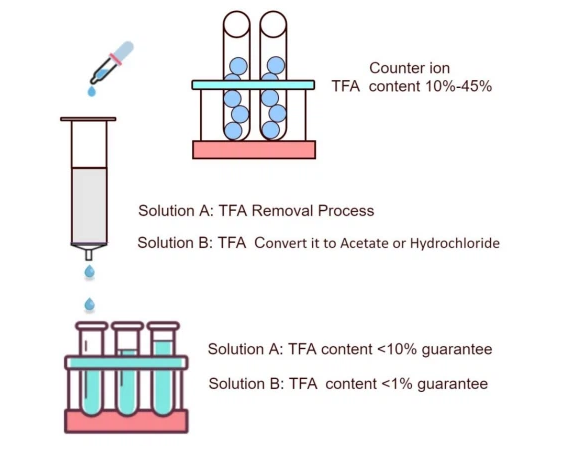
Peptides play a crucial role in various fields, including pharmaceuticals, biotechnology, and medical research. However, the synthesis of peptides often involves the use of trifluoroacetic acid (TFA), which serves as a key reagent in peptide purification. While effective, residual TFA can lead to instability and toxicity, impacting the overall quality and performance of peptides. To address this issue, specialised purification techniques are essential to ensure the highest purity levels in peptide formulations. This is where Peptide TFA Removal Service becomes a vital process in research and commercial applications.
The Need for TFA Removal in Peptides
TFA is widely used in peptide synthesis due to its strong acid properties, which facilitate peptide cleavage from resin and improve solubility. However, the presence of TFA salts in peptides can create unwanted effects, such as altering biological activity, reducing shelf stability, and increasing cytotoxicity. Many regulatory agencies and research institutions recommend the removal of TFA to achieve higher standards in peptide applications. A reliable purification method ensures that peptides remain biologically active, stable, and compatible with pharmaceutical-grade formulations.
How Peptide TFA Removal Works
The Peptide TFA Removal Service involves specialized purification techniques that effectively replace TFA with safer counterions. This is achieved through various methods, including:
● Ion Exchange Chromatography: This technique helps in substituting TFA with alternative counterions such as acetate, chloride, or phosphate to enhance peptide stability.
● Lyophilization and Reconstitution: By freeze-drying peptides and reconstituting them in a suitable buffer, researchers can remove TFA remnants and obtain purer formulations.
● Reverse-Phase HPLC Processing: High-performance liquid chromatography (HPLC) is commonly used to separate TFA from peptide sequences, ensuring minimal contamination.
These methods ensure that peptides maintain their structural integrity while meeting research and industrial standards.
Benefits of TFA-Free Peptides
● Improved Biological Activity: Peptides without residual TFA demonstrate enhanced interaction with target receptors, improving research outcomes.
● Reduced Toxicity Risks: Removing TFA minimizes potential side effects, making peptides safer for pharmaceutical and biotechnological applications.
● Enhanced Peptide Stability: Peptides free from TFA degradation products exhibit longer shelf life and increased solubility.
● Regulatory Compliance: Purified peptides align with industry standards, making them suitable for clinical trials and therapeutic use.
Achieving high-purity peptides is essential for advancing scientific research and pharmaceutical development. Utilizing a Peptide TFA Removal Service ensures that peptides meet the necessary quality standards, eliminating impurities that could impact performance and safety. For researchers and businesses looking to enhance peptide purity, Biorunstar offers reliable and efficient TFA removal solutions.